Diagnostic Services Manitoba Year In Review 2021
Senior Staff and Contact Information
|
Red Cell Serology Laboratory Medical Director: Charles Musuka, MD, FRCPC |
Platelet Immunology Laboratory Medical Director Akash Gupta, MD, FRCPC204-789-1125 akash.gupta@blood.ca |
|
Diagnostic Services Manager Dora Lopes-Carvalho, MLT |
Technical Supervisor, Testing Lynne Meilleur, MLT |
|
Charge Technologists: |
|
|
Red Cell Serology: Sherry Watt, MLT |
Red Cell Serology: Henri Beaubien, MLT |
|
Platelet Immunology Laboratory Jacqueline Wong, MLT |
Perinatal Laboratory Phone # 204-789-1088 |
|
Crossmatch / Accession Laboratory Phone # 204-789-1085 |
Platelet Immunology Laboratory Phone # 204-789-1152 |
|
Diagnostic Services Website |
https://blood.ca/en/hospital-services |
Figures
- Figure 1: Total Perinatal Specimens Tested
- Figure 2: Total Number of Perinatal Antibodies
- Figure 3: Frequency of Clinically Significant Antibodies
- Figure 4: Total Crossmatch Specimens Tested
- Figure 5: Total Number of Crossmatch Antibodies
- Figure 6: Frequency of Clinically Significant Antibodies 2021
- Figure 7: Total Platelet Immunology Donor Specimens Tested
- Figure 8: Total Platelet Immunology Patient Specimens Tested
- Figure 9: Rh D Testing Algorithm
- Figure 10: Perinatal Rejection Reasons
- Figure 11: Crossmatch Rejection Reasons 2021
- Figure 12: Platelet Immunology Rejection Reasons 2021
TABLES
- Table 1: Total Number of Perinatal Specimens and Patients Tested between 2017 and 2021
- Table 2: Total Number of Clinically Significant and Insignificant Perinatal Antibodies Detected between 2017 and 2021
- Table 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
- Table 4: Perinatal Patient Antibody Titres Performed in Year 2021 and changed from Non-critical Level to Critical Level
- Table 5: Combination Antibodies Detected in Perinatal Patients in 2021
- Table 6: Total Crossmatch/Reference Specimens Tested by Red Cell Serology Laboratory between 2017 and 2021
- Table 7: Total Number of Crossmatch Antibodies Detected between 2017 and 2021
- Table 8: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
- Table 9: Platelet Immunology Tests Performed
- Table 10: Patient # - RHD Type/Result 2021
- Table 11: Turnaround Time – Routine Criteria by Specimen Type
- Table 12: Turnaround Time – Routine Perinatal Specimens between 2017 and 2021
- Table 13: Turnaround Time – Routine Crossmatch Specimens between 2017 and 2021
- Table 14: Turnaround Time – Reference Specimens between 2017 and 2021
- Table 15: Turnaround Time - Platelet Immunology Specimens between 2017 and 2021
- Table 16: Quarterly Rejection Rates – Perinatal Specimens 2021
- Table 17: Quarterly Rejection Rates – Crossmatch Specimens 2021
- Table 18: Quarterly Rejection Rates – Platelet Immunology Specimens (Patient and Donor) 2021
Perinatal Laboratory
The Perinatal Laboratory within Diagnostic Services at Canadian Blood Services provides diagnostic testing of perinatal samples for blood type and red blood cell antibodies. Results from this screening assist physicians, midwives and nurse practitioners in ensuring the appropriate management of a pregnancy for both the patient and baby.
Testing Performed
Canadian Blood Services Perinatal Laboratory routinely performs the following tests:
- ABO/Rh blood type
- Screen for red blood cell antibodies
- Antibody Identification, if antibodies are detected
- Antibody Identification referrals
- Antibody Titration, if a clinically significant antibody is identified
- Phenotyping
- Fetal Bleed Screening Test
- Kleihauer-Betke Test for Quantitation of fetal-maternal hemorrhage
- Direct Antiglobulin Test for detection of HDFN (Hemolytic Disease of the Fetus/Newborn)
- Bedside testing during fetal cordocentesis
Automated ABO/Rh and Antibody Screen assays are routinely performed on the Immucor NEO Iris analyzer (hemagglutination and solid phase testing). A combination of solid phase testing and indirect antiglobulin tube testing using PEG for enhancement are the primary antibody identification methods.
Testing Frequency
Prenatal – Initial Testing: All patients should be tested upon their first prenatal visit.
Prenatal – 26-28 Weeks Gestation: All Rh-negative patients should be retested at 26-28 weeks gestation. Rh positive patients should also be retested at 26-28 weeks gestation when there is only one blood group result available (usually first pregnancy) or if patient is at increased risk of allo-immunization (e.g. previous transfusion, maternal trauma, obstetrical procedure or suspected fetal hemorrhage).
Prenatal– Antibody Present: If the antibody is known to cause HDFN, it is recommended that specimens be submitted every two to four weeks for the duration of the pregnancy dependant on the specificity of the antibody and the strength of the antibody titre. More frequent testing may be indicated if the antibody titre rises rapidly or if clinical monitoring mandates that additional sampling would provide helpful information. Less frequent sampling may also be recommended for antibodies that are unlikely to be clinically significant or in cases where clinical monitoring through fetal doppler ultrasound has commenced.
Postnatal: Following delivery, specimens from the patientand her baby should be tested if the Rh of the patient is unknown, the patient is Rh negative, the patient has a clinically significant antibody or if the baby shows signs of HDFN (i.e. anemia or jaundice). Midwives or hospitals that do not perform transfusion medicine testing should submit specimens to Canadian Blood Services. A fetal bleed screening test is performed if a Rh-negative patientdelivers a Rh-positive baby. The Kleihauer-Betke assay is performed when the patient has a positive fetal bleed screening test.
Newborns (Cords): Cord blood or neonate specimens must be submitted with the postnatal specimen as noted above. ABO/Rh testing is performed on cord or neonatal, when indicated, on specimens submitted to Canadian Blood Services. The direct antiglobulin test is performed if the perinatal patient has a clinically significant antibody or on request if the baby shows signs of HDFN (i.e. anemia or jaundice). This is especially important when the perinatal patient is Rh negative or when the perinatal patient has a clinically significant antibody. If the baby has unexpected anemia or jaundice, assessment of the cord blood sample for blood group and DAT may also be helpful.
Partners: When a perinatal patient has an antibody capable of causing HDFN, specimens from the partner will be requested for ABO/Rh and antigen phenotyping. This will assist in assessing the probability of the baby being affected by the antibody. Partners’ specimens may also be tested to assess Rh Immune Globulin (RhIG) eligibility of Rh negative perinatal patients.
Specimens Tested
The data includes all perinatal patient tested, including referral patients from other provincial jurisdictions. The total number of specimens tested shows a slight decrease when compared to the last 4 years as seen in Table 1 below. The data in this report reflects a calendar year period to enable better correlation to other government statistical data (Statistics Canada birth statistics).
Figure 1: Total Number of Perinatal Specimens Tested between 2017 and 2021

Table 1: Total Number of Perinatal Specimens and Patients Tested between 2017 and 2021
|
Specimen Type |
Test Type |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|---|
|
Perinatal |
Perinatal- Type and Screen |
31,800 |
31,123 |
29,361 |
26,769 |
25,652 |
|
Partner |
Partner- ABO/Rh |
388 |
358 |
364 |
310 |
280 |
|
Cord- ABO/Rh |
Cord- ABO/Rh |
2,111 |
2,474 |
2,625 |
2,906 |
1,733 |
|
Total # of Specimens Tested |
|
34,299 |
33,955 |
32,350 |
29,985 |
27,665 |
|
Total # of Patients Tested |
|
24,248 |
24,079 |
23,360 |
24,793 |
22,393 |
Text Version – Table 1
Total number of perinatal specimens and patients tested between 2017 and 2021 includes specimen types Perinatal, Partner and Cord. Perinatal specimens were tested for Type and Screen. Total number of perinatal specimens tested in 2017 were 31800, in 2018 – number of perinatal specimens were 31123, in 2019 – number of perinatal specimens were 29361, in 2020 – number of perinatal specimens were 26769 and in 2021, number of perinatal specimens were 25652. Partner specimens were tested for ABO/Rh. Total number of partner specimens tested in 2017 were 388, in 2018 – number of partner specimens were 358, in 2019 – number of partner specimens were 364, in 2020 – number of partner specimens were 310 and in 2021, number of perinatal specimens were 280. Cord specimens were also tested for ABO/Rh. Total number of cord specimens tested in 2017 were 2111, in 2018 – number of cord specimens were 2474, in 2019 – number of cord specimens were 2625, in 2020 – number of cord specimens were 2906 and in 2021, number of cord specimens were 1733. Total number of patients tested in 2017 were 24248, in 2018 – total number of patients tested were 24079, in 2019 – total number of patients tested were 23360, in 2020 – total number of patients tested were 24793 and a total of 22393 patients were tested in 2021.
Antibodies Identified
In 2021, a total of 192 antibodies were reported (see Table 2). This is slightly less than 2020 where 212 antibodies were reported. One hundred and eighty patients had antibodies identified during their pregnancies (slightly decreased from 206 patients in 2020). Breakdown of antibodies identified within the 192 patients consisted of 183 clinically significant antibodies and 9 clinically insignificant antibodies. Twenty-four patients had multiple clinically significant antibodies. Passive anti-D data has been excluded from the preceding numbers.
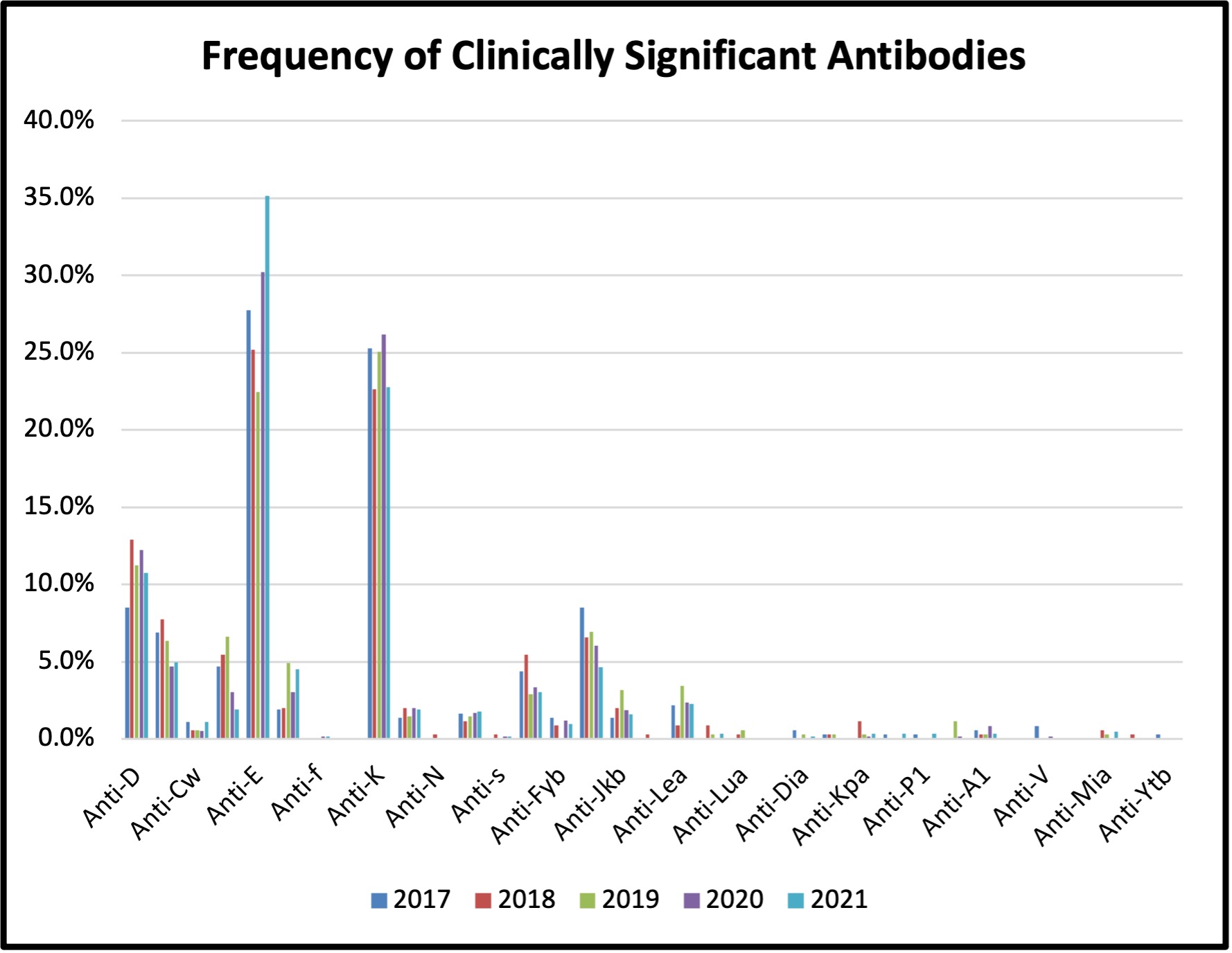
Antibodies identified were considered clinically significant if they have been reported to cause HDFN. The most common clinically significant antibodies identified were anti-E, anti-K, anti-C, anti-M, and antiD, (see Figure 3) which together represented 81.5% of the total antibodies identified.
Titres for 5 of the clinically significant antibodies increased from non-critical to critical levels during the pregnancy with a total of 20 antibody titres at critical levels (see Table 3). Recommendations were made for all patients with a critical titre level (current or previous pregnancy) and all Kell system antibodies to be referred to a High Risk Fetal Assessment Clinic for further follow-up and monitoring during pregnancy.
Figure 2: Total Number of Clinically Significant Perinatal Antibodies Detected between 2017 and 2021

Table 2: Total Number of Clinically Significant and Insignificant Perinatal Antibodies Detected between 2017 and 2021
Perinatal Antibodies Identified 2021 |
|||||
|---|---|---|---|---|---|
|
Clinically Significant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|
Anti-D |
11 |
11 |
16 |
19 |
11 |
|
Anti-C |
9 |
10 |
6 |
10 |
21 |
|
Anti-Cw |
2 |
0 |
0 |
0 |
0 |
|
Anti-Ce |
0 |
0 |
0 |
1 |
1 |
|
Anti-ce |
0 |
1 |
0 |
0 |
0 |
|
Anti-c |
20 |
12 |
11 |
6 |
5 |
|
Anti-E |
66 |
64 |
69 |
74 |
68 |
|
Anti-e |
8 |
6 |
6 |
2 |
4 |
|
Anti-f |
0 |
1 |
0 |
0 |
0 |
|
Anti-G |
1 |
2 |
3 |
3 |
2 |
|
Anti-Ge3 |
0 |
1 |
0 |
0 |
1 |
|
Anti-K |
56 |
58 |
51 |
43 |
36 |
|
Anti-Kpa |
1 |
0 |
1 |
0 |
0 |
|
Anti-Kpb |
1 |
0 |
0 |
0 |
0 |
|
Anti Lua |
1 |
0 |
0 |
0 |
0 |
|
Anti-Lub |
0 |
0 |
1 |
0 |
0 |
|
Anti-M* |
18 |
16 |
14 |
18 |
13 |
|
Anti-S |
6 |
7 |
6 |
6 |
7 |
|
Anti-s |
2 |
0 |
1 |
1 |
0 |
|
Anti-Fya |
4 |
4 |
2 |
4 |
2 |
|
Anti-Fyb |
4 |
4 |
1 |
2 |
0 |
|
Anti-Jka |
20 |
17 |
13 |
17 |
6 |
|
Anti-Jkb |
4 |
4 |
1 |
4 |
4 |
|
Anti-Jk3 |
1 |
0 |
0 |
0 |
0 |
|
Anti-Dia |
0 |
1 |
1 |
0 |
0 |
|
Anti-Dib |
0 |
1 |
1 |
0 |
0 |
|
Anti Mia |
0 |
1 |
0 |
0 |
0 |
|
Anti-U |
0 |
0 |
0 |
0 |
1 |
|
Anti-V |
1 |
1 |
0 |
0 |
0 |
|
Anti-Wra |
2 |
1 |
2 |
2 |
1 |
|
Total |
238 |
223 |
206 |
212 |
183 |
*Anti-M – IgG antibody detected
|
Clinically Insignificant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-A1 |
1 |
0 |
0 |
0 |
0 |
|
Anti-He |
1 |
0 |
0 |
0 |
0 |
|
Anti-JMH |
1 |
1 |
1 |
0 |
1 |
|
Anti-Lea |
11 |
17 |
13 |
8 |
7 |
|
2 |
2 |
1 |
0 |
0 |
|
|
Anti-N |
2 |
1 |
1 |
0 |
0 |
|
Anti-P1 |
3 |
2 |
0 |
1 |
1 |
|
Passive Anti-D (not included in totals) |
813 |
763 |
665 |
248 |
212 |
|
TOTAL: Clinically Insignificant Antibodies |
21 |
23 |
16 |
9 |
9 |
Figure 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021

Table 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
|
|
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-D |
4.6% |
4.9% |
7.8% |
9.0% |
6.0% |
|
Anti-C |
3.8% |
4.5% |
2.9% |
4.7% |
11.5% |
|
Anti-Cw |
0.8% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Ce |
0.0% |
0.0% |
0.0% |
0.5% |
0.5% |
|
Anti-ce |
0.0% |
0.4% |
0.0% |
0.0% |
0.0% |
|
Anti-c |
8.4% |
5.4% |
5.3% |
2.8% |
2.7% |
|
Anti-E |
27.7% |
28.7% |
33.5% |
34.9% |
37.2% |
|
Anti-e |
3.4% |
2.7% |
2.9% |
0.9% |
2.2% |
|
Anti-f |
0.0% |
0.4% |
0.0% |
0.0% |
0.0% |
|
Anti-G |
0.4% |
0.9% |
1.5% |
1.4% |
1.1% |
|
Anti-Ge3 |
0.0% |
0.4% |
0.0% |
0.0% |
0.5% |
|
Anti-K |
23.5% |
26.0% |
24.8% |
20.3% |
19.7% |
|
Anti-Kpa |
0.4% |
0.0% |
0.5% |
0.0% |
0.0% |
|
Anti-Kpb |
0.4% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti Lua |
0.4% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Lub |
0.0% |
0.0% |
0.5% |
0.0% |
0.0% |
|
Anti-M* |
7.6% |
7.2% |
6.8% |
8.5% |
7.1% |
|
Anti-S |
2.5% |
3.1% |
2.9% |
2.8% |
3.8% |
|
Anti-s |
0.8% |
0.0% |
0.5% |
0.5% |
0.0% |
|
Anti-Fya |
1.7% |
1.8% |
1.0% |
1.9% |
1.1% |
|
Anti-Fyb |
1.7% |
1.8% |
0.5% |
0.9% |
0.0% |
|
Anti-Jka |
8.4% |
7.6% |
6.3% |
8.0% |
3.3% |
|
Anti-Jkb |
1.7% |
1.8% |
0.5% |
1.9% |
2.2% |
|
Anti-Jk3 |
0.4% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Dia |
0.0% |
0.4% |
0.5% |
0.0% |
0.0% |
|
0.0% |
0.4% |
0.5% |
0.0% |
0.0% |
|
|
Anti Mia |
0.0% |
0.4% |
0.0% |
0.0% |
0.0% |
|
Anti-V |
0.4% |
0.4% |
0.0% |
0.0% |
0.0% |
|
Anti-Wra |
0.8% |
0.4% |
0.8% |
0.9% |
0.5% |
Table 4: Perinatal Patient Antibody Titres Performed in Year 2021 and changed from Non-critical Level to Critical Level
|
Antibody |
Critical Level |
Non-Critical Level |
Non-Critical to Critical |
|---|---|---|---|
|
Anti-D |
3 |
10 |
2 |
|
Anti-C |
0 |
5 |
0 |
|
Anti-E |
3 |
44 |
2 |
|
Anti-c |
1 |
4 |
0 |
|
Anti-e |
0 |
2 |
0 |
|
Anti-DC |
0 |
1 |
0 |
|
Anti-Ec |
1 |
4 |
1 |
|
Anti-Ce |
0 |
1 |
0 |
|
Anti-CG |
0 |
1 |
0 |
|
Anti-K* |
2 |
3 |
0 |
|
Anti-Fya |
0 |
2 |
0 |
|
Anti-Fyb |
0 |
0 |
0 |
|
Anti-Jka |
0 |
6 |
0 |
|
0 |
1 |
0 |
|
|
Anti-M |
0 |
9 |
0 |
|
Anti-S |
0 |
4 |
0 |
|
Anti-s |
0 |
0 |
0 |
*Note: Anti-K is considered critical at any titre. Antibody titres for Kell system antibodies may be performed in Manitoba after consultation with the Medical Officer
Table 5: Combination Antibodies Detected in Perinatal Patients in 2021
|
Combination Antibodies |
Total |
|---|---|
|
Anti-C, Anti-E |
0 |
|
Anti-C, Anti-e |
2 |
|
Anti-c, Anti-E, Anti-Jka |
1 |
|
Anti-c, Anti-Jka |
1 |
|
Anti-C, Anti-G |
2 |
|
Anti-c, Anti-K |
2 |
|
Anti-C, Anti-M |
1 |
|
Anti-C, Anti-Jka |
1 |
|
Anti-D, Anti-M |
1 |
|
Anti-D, Anti-C, Anti-G |
0 |
|
Anti-E, Anti-c |
3 |
|
Anti-E, Anti-c, Anti-K, Anti-K, Anti-Fya |
1 |
|
Anti-C, Anti-K, Anti-S |
1 |
|
Anti-E, Anti-Fya |
1 |
|
Anti-E, Anti-Jka |
3 |
|
Anti-E, Anti-K |
2 |
|
0 |
|
|
Anti-K, Anti-Jka |
1 |
|
Anti-Fya, Anti-Jkb |
1 |
|
Total |
24 |
Crossmatch / Reference Laboratory
The Crossmatch/ Reference Laboratory Winnipeg Red Cell Serology, Diagnostic Services provides centralized transfusion medicine services and testing to approximately 70 hospitals in Manitoba and eastern Nunavut that do not perform these tests. Reference services are provided for 4 rural hospitals with crossmatching laboratories in Manitoba and 12 hospitals in Northwest Ontario. Hospital patients who are repeatedly transfused may develop red cell antibodies and as a result may have difficulty in tolerating transfusions. Diagnostic Services has specialized and experienced technologists that assist and provide consultation to hospital transfusion medicine laboratories (24 hours, 7 days per week). The Reference Laboratory identifies red cell antibodies and provides transfusion recommendations. Diagnostic Services has a varied selection of specialized procedures and rare reagents to resolve more difficult red cell antibody cases. Staff within our department may collaborate with other references laboratories such as the National Immunohematology Reference laboratory (NIRL).
Diagnostic Services Red Cell Antibody Investigations
In 2021, hospitals have referred 146 requests for red cell antibody identification.
Referring hospitals have different capabilities and expertise in resolving red cell antibody investigations.
Canadian Blood Services, Diagnostic Services provides consultation and testing support including antibody investigation, advanced or alternative techniques where required, and recommendations for compatibility testing methods and selection of appropriate donor unit phenotypes if necessary.
Reporting may include interim, final and supplemental reports, depending on the urgency of the testing, the need for patient transfusion and the complexity of investigation. When a new antibody is identified by the Diagnostic Services laboratory a patient wallet card may be provided.
Testing Performed
The Crossmatch/Reference Laboratory routinely performs the following tests:
- ABO/Rh blood type
- Screen for red blood cell antibodies
- Antibody Identification, if antibodies are detected
- Crossmatch, electronic and serological
- Isohemagglutinin Titre
- Phenotyping (patient and donor units)
- Transfusion Reaction Investigation
- Direct Antiglobulin Test
- Elution and Absorption
- Cold Agglutinin Screen
- Thermal Amplitude
Antibody Screening is routinely performed by solid phase testing. A combination of solid phase testing and indirect antiglobulin tube testing using PEG for enhancement are the primary antibody identification methods. PEG IAT is also the manual back-up method for antibody screening.
The Crossmatch Laboratory distributes both stock and crossmatched red cell and platelet components to those hospitals which receive all of their transfusion medicine services from Canadian Blood Services.
The Crossmatch Laboratory performs complex antibody investigations and distributes crossmatch compatible (or least incompatible) red cell units.
Specimens Tested.
The data in this report reflects a calendar year period to enable better correlation to other government statistical data (ie: Statistics Canada birth statistics).
The total number of crossmatch specimens tested has remained consistent over the last 4 years as illustrated in Table 6 below. The implementation of the Trace Line laboratory information system (LIS) was completed at 16 hospitals in Winnipeg and rural Manitoba in 2015. These hospitals now hold a stock inventory of red blood cell components and perform electronic crossmatch on demand; thus, reducing the number of red blood cells issued and reserved for specific patients on hand in the hospital Blood Bank. The number of red blood cell components distributed has stabilized as hospitals appear to have adjusted inventories to optimal levels. As part of Choosing Wisely Canada, a “Just One” campaign that highlighted “Why give two when one will do?” was rolled out in late 2018 at the Winnipeg tertiary care facilities which may have contributed to the reduction in red blood cell utilization.
The spike in number of product transformation is a result of the implementation of Red Cell aliquots in January 2020. The lab will prepare and provide small volume red cell aliquots for neonatal and pediatric transfusion.
Figure 4: Total Crossmatch/Reference Specimens Tested by Red Cell Serology Laboratory between 2017 and 2021
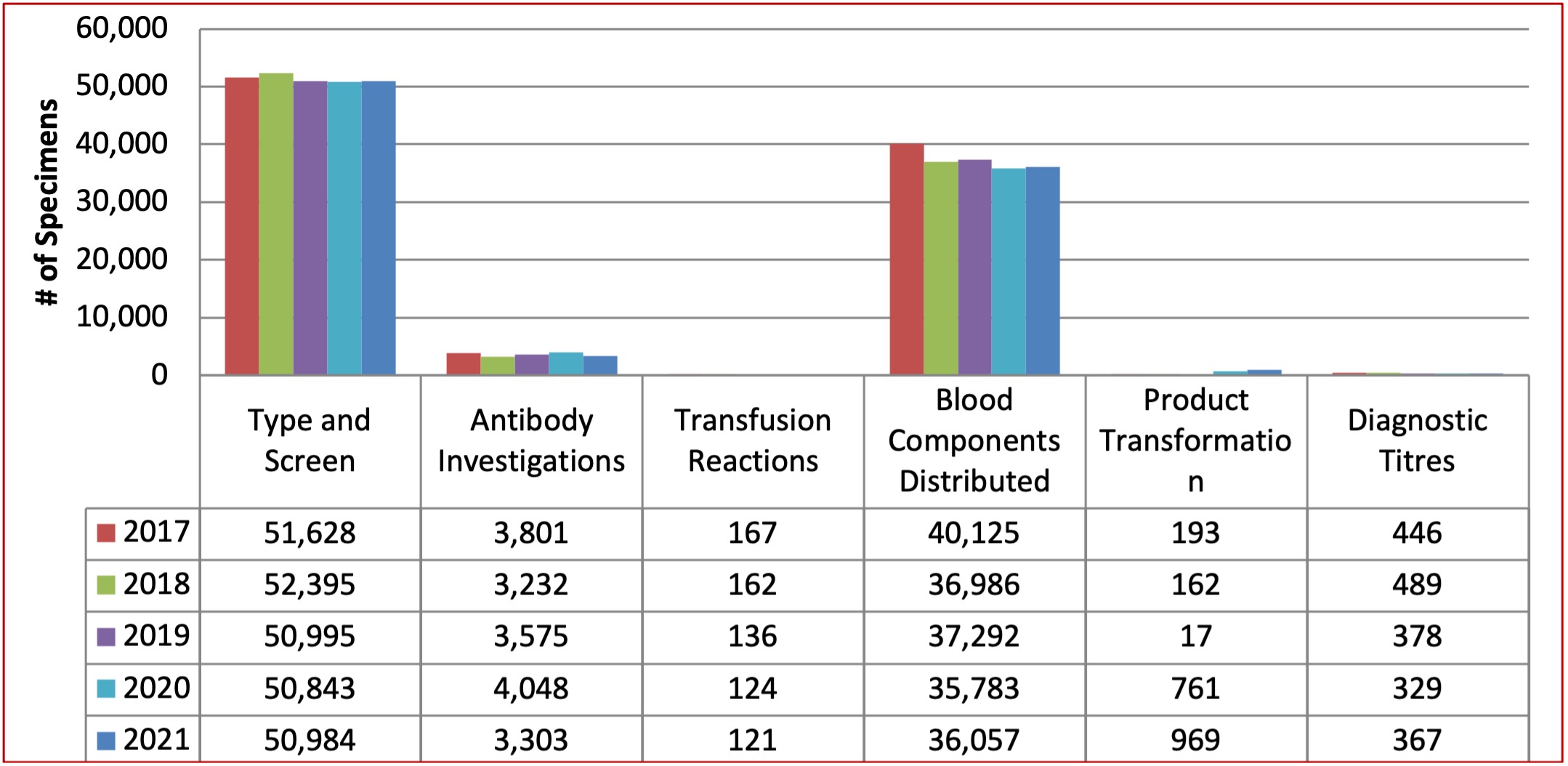
Table 6: Total Crossmatch/Reference Specimens Tested by Red Cell Serology Laboratory between 2017 and 2021
|
Specimen Type |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Type and Screen |
51,628 |
52,395 |
50,995 |
50,843 |
50,984 |
|
Antibody Investigations |
3,801 |
3,232 |
3,575 |
4,048 |
3,303 |
|
Transfusion Reactions |
167 |
162 |
136 |
124 |
121 |
|
Blood Components Distributed |
40,125 |
36,986 |
37,292 |
35,783 |
36,057 |
|
193 |
162 |
17 |
761 |
969 |
|
|
Diagnostic Titres |
446 |
489 |
378 |
329 |
367 |
|
Test Totals (excluding components distributed) |
56,235 |
56,440 |
55,101 |
56,105 |
55,744 |
|
Patients Tested |
30,553 |
31,025 |
29,528 |
27,617 |
28,020 |
Antibodies Identified
In 2021, total of 1031 antibodies clinically significant and insignificant antibodies were reported (see Table 7). The distribution of the most common antibodies remains consistent. Six hundred and twenty-three patients had antibodies identified, of these; 197 patients had multiple antibodies.
The difference in number of antibodies detected since 2019 is a reflective of a process change in reporting made in the lab in February 2020 and may not represent a significant increase in the rate of antibodies being detected. This change in process did not affect the reporting of Perinatal antibodies, therefore the jump in number is not observed in that patient population. Antibodies will continue to be reported in this manner in Winnipeg for the foreseeable future and preliminary numbers suggest that 2021 will show consistency with what is reported in 2020.
Antibodies identified were considered clinically significant if they have been reported to cause acute or delayed hemolytic transfusion reactions. The most common clinically significant antibodies identified were: anti-E, anti-K, anti-D, anti-Jka, anti-c, anti-C, and anti-e, (see Figure 5).
Figure 5: Total Number of Crossmatch Antibodies Detected between 2017 and 2021
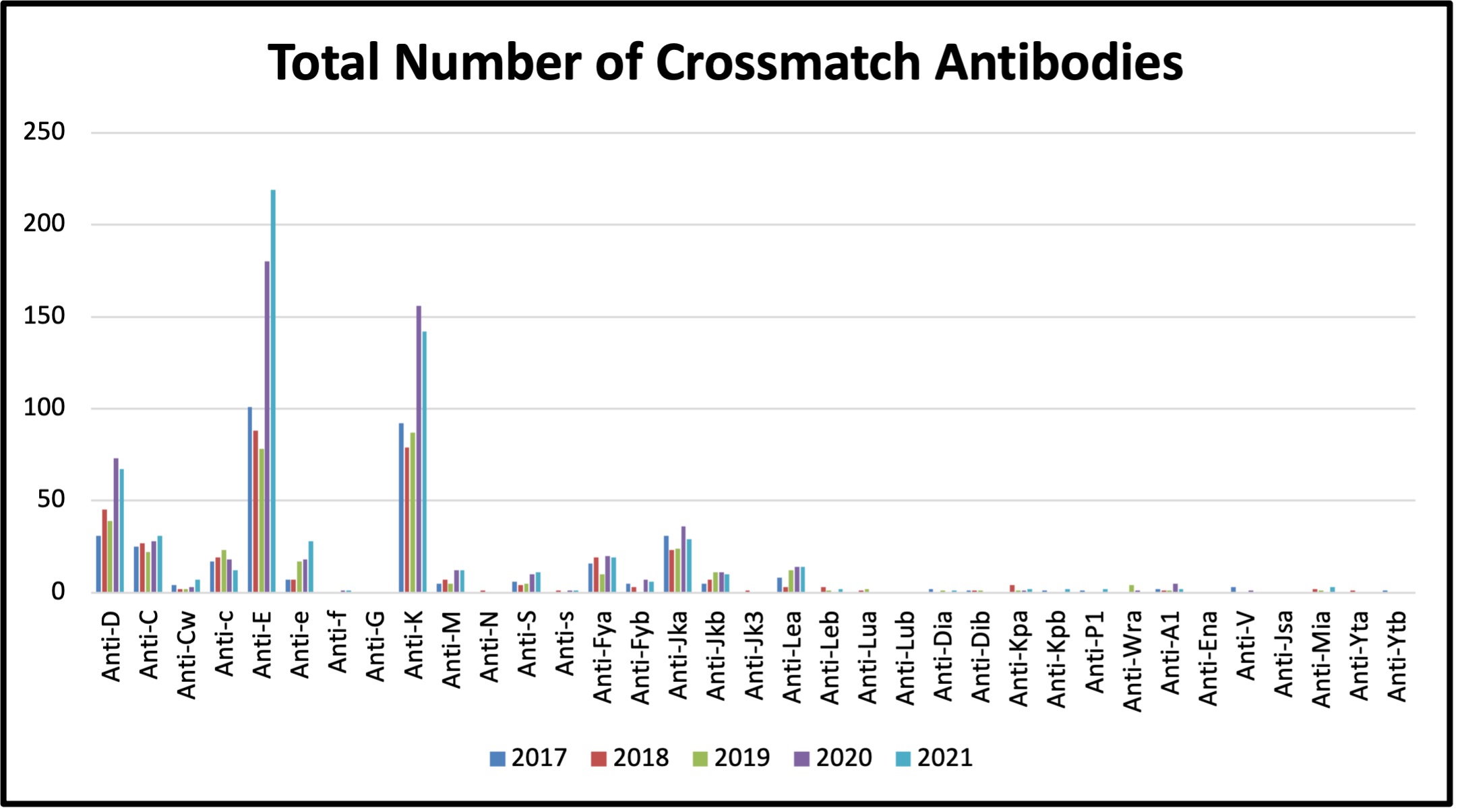
Table 7: Total Number of Crossmatch Antibodies Detected between 2017 and 2021
|
Crossmatch Antibodies Identified 2021 |
|||||
|---|---|---|---|---|---|
|
Clinically Significant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|
Anti-D |
31 |
45 |
39 |
73 |
67 |
|
Anti-C |
25 |
27 |
22 |
28 |
31 |
|
Anti-Cw |
4 |
2 |
2 |
3 |
7 |
|
Anti-c |
17 |
19 |
23 |
18 |
12 |
|
Anti-E |
101 |
88 |
78 |
180 |
219 |
|
Anti-e |
7 |
7 |
17 |
18 |
28 |
|
Anti-f |
0 |
0 |
0 |
1 |
1 |
|
Anti-G |
0 |
0 |
0 |
0 |
0 |
|
Anti-K |
92 |
79 |
87 |
156 |
142 |
|
Anti-M |
5 |
7 |
5 |
12 |
12 |
|
Anti-N |
0 |
1 |
0 |
0 |
0 |
|
Anti-S |
6 |
4 |
5 |
10 |
11 |
|
Anti-s |
0 |
1 |
0 |
1 |
1 |
|
Anti-Fya |
16 |
19 |
10 |
20 |
19 |
|
Anti-Fyb |
5 |
3 |
0 |
7 |
6 |
|
Anti-Jka |
31 |
23 |
24 |
36 |
29 |
|
Anti-Jkb |
5 |
7 |
11 |
11 |
10 |
|
Anti-Jk3 |
0 |
1 |
0 |
0 |
0 |
|
Anti-Lea |
8 |
3 |
12 |
14 |
14 |
|
Anti-Leb |
0 |
3 |
1 |
0 |
2 |
|
Anti-Lua |
0 |
1 |
2 |
0 |
0 |
|
Anti-Lub |
0 |
0 |
0 |
0 |
0 |
|
Anti-Dia |
2 |
0 |
1 |
0 |
1 |
|
Anti-Dib |
1 |
1 |
1 |
0 |
0 |
|
Anti-Kpa |
0 |
4 |
1 |
1 |
2 |
|
Anti-Kpb |
1 |
0 |
0 |
0 |
2 |
|
Anti-P1 |
1 |
0 |
0 |
0 |
2 |
|
Anti-Wra |
0 |
0 |
4 |
1 |
0 |
|
Anti-A1 |
2 |
1 |
1 |
5 |
2 |
|
Anti-Ena |
0 |
0 |
0 |
0 |
0 |
|
Anti-V |
3 |
0 |
0 |
1 |
0 |
|
Anti-Jsa |
0 |
0 |
0 |
0 |
0 |
|
0 |
2 |
1 |
0 |
3 |
|
|
Anti-Yta |
0 |
1 |
0 |
0 |
0 |
|
Anti-Ytb |
1 |
0 |
0 |
0 |
0 |
|
Total |
364 |
349 |
347 |
596 |
623 |
* The difference in number of antibodies noted since 2019 is a reflective of a process change in reporting and may not represent a significant increase in the rate of antibodies being detected.
Figure 6: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
Table 8: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
|
Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-D |
8.5% |
12.9% |
11.2% |
12.2% |
10.8% |
|
Anti-C |
6.9% |
7.7% |
6.3% |
4.7% |
5.0% |
|
Anti-Cw |
1.1% |
0.6% |
0.6% |
0.5% |
1.1% |
|
Anti-c |
4.7% |
5.4% |
6.6% |
3.0% |
1.9% |
|
Anti-E |
27.7% |
25.2% |
22.5% |
30.2% |
35.2% |
|
Anti-e |
1.9% |
2.0% |
4.9% |
3.0% |
4.5% |
|
Anti-f |
0.0% |
0.0% |
0.0% |
0.2% |
0.2% |
|
Anti-G |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-K |
25.3% |
22.6% |
25.1% |
26.2% |
22.8% |
|
Anti-M |
1.4% |
2.0% |
1.4% |
2.0% |
1.9% |
|
Anti-N |
0.0% |
0.3% |
0.0% |
0.0% |
0.0% |
|
Anti-S |
1.6% |
1.1% |
1.4% |
1.7% |
1.8% |
|
Anti-s |
0.0% |
0.3% |
0.0% |
0.2% |
0.2% |
|
Anti-Fya |
4.4% |
5.4% |
2.9% |
3.4% |
3.0% |
|
Anti-Fyb |
1.4% |
0.9% |
0.0% |
1.2% |
1.0% |
|
Anti-Jka |
8.5% |
6.6% |
6.9% |
6.0% |
4.7% |
|
Anti-Jkb |
1.4% |
2.0% |
3.2% |
1.8% |
1.6% |
|
Anti-Jk3 |
0.0% |
0.3% |
0.0% |
0.0% |
0.0% |
|
Anti-Lea |
2.2% |
0.9% |
3.5% |
2.3% |
2.2% |
|
Anti-Leb |
0.0% |
0.9% |
0.3% |
0.0% |
0.3% |
|
Anti-Lua |
0.0% |
0.3% |
0.6% |
0.0% |
0.0% |
|
Anti-Lub |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Dia |
0.5% |
0.0% |
0.3% |
0.0% |
0.2% |
|
Anti-Dib |
0.3% |
0.3% |
0.3% |
0.0% |
0.0% |
|
Anti-Kpa |
0.0% |
1.1% |
0.3% |
0.2% |
0.3% |
|
Anti-Kpb |
0.3% |
0.0% |
0.0% |
0.0% |
0.3% |
|
Anti-P1 |
0.3% |
0.0% |
0.0% |
0.0% |
0.3% |
|
Anti-Wra |
0.0% |
0.0% |
1.2% |
0.2% |
0.0% |
|
Anti-A1 |
0.5% |
0.3% |
0.3% |
0.8% |
0.3% |
|
Anti-Ena |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-V |
0.8% |
0.0% |
0.0% |
0.2% |
0.0% |
|
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
|
Anti-Mia |
0.0% |
0.6% |
0.3% |
0.0% |
0.5% |
|
Anti-Yta |
0.0% |
0.3% |
0.0% |
0.0% |
0.0% |
|
Anti-Ytb |
0.3% |
0.0% |
0.0% |
0.0% |
0.0% |
Platelet Immunology Laboratory
The Platelet Immunology Laboratory within Diagnostic Services at Canadian Blood Services provides human leukocyte (HLA) and platelet specific (HPA) antigen typing and antibody investigation testing to assist health care providers in the management of thrombocytopenic patients who have become refractory to vital platelet transfusions, patients affected by neonatal alloimmune thrombocytopenia and autoimmune disorders and patients suspected to have had platelet antibody mediated adverse transfusion events such as post transfusion purpura (PTP). The Laboratory also performs testing on patients and donors for the investigation of Transfusion Related Acute Lung Injury (TRALI). The Laboratory provides service to all Manitoba hospitals and is a national reference lab for any hospital in Canada requiring these testing services.
In addition, the Laboratory also performs HLA and HPA typing on blood donors prior to being placed onto a national platelet donor registry. The registry is used to conduct searches to identify suitably compatible donors who can be used for patients that show no benefit from conventional platelet components.
Testing Performed
The Platelet Immunology Laboratory routinely performs the following tests:
- HLA Antigen Typing
- HLA Antibody Screen
- HLA Antibody Identification, if antibodies are detected
- HLA Antigen Typing for disease association
- HPA Typing
- HPA Screening
- HPA Antibody Identification, if antibodies are detected
- Platelet Crossmatch
- Selection of HLA/HPA Compatible Donors for Platelet Transfusion
HLA antibody screening and identification is performed using Luminex bead technology. Whereas HPA antibody screening, identification and crossmatching are performed using a solid phase platform, commercial ELISA kits and the MAIPA method.
A combination of Luminex® multiplex technology, Bioarray eMAP® (Elongation-mediated Multiplexed Analysis of Polymorphisms) technology and/or MicroSSP are the primary HLA and HPA genotyping methods utilized for genotyping both patients and donors.
Selection lists of HLA/HPA compatible donors for patients’ requiring platelet transfusion support are generated by the Platelet Immunology Lab using the national platelet donor database.
Specimens Tested
Table 9 below illustrates the total number of Platelet Immunology specimens tested.
Figure 7: Total Platelet Immunology Donor Specimens Tested between 2017 and 2021
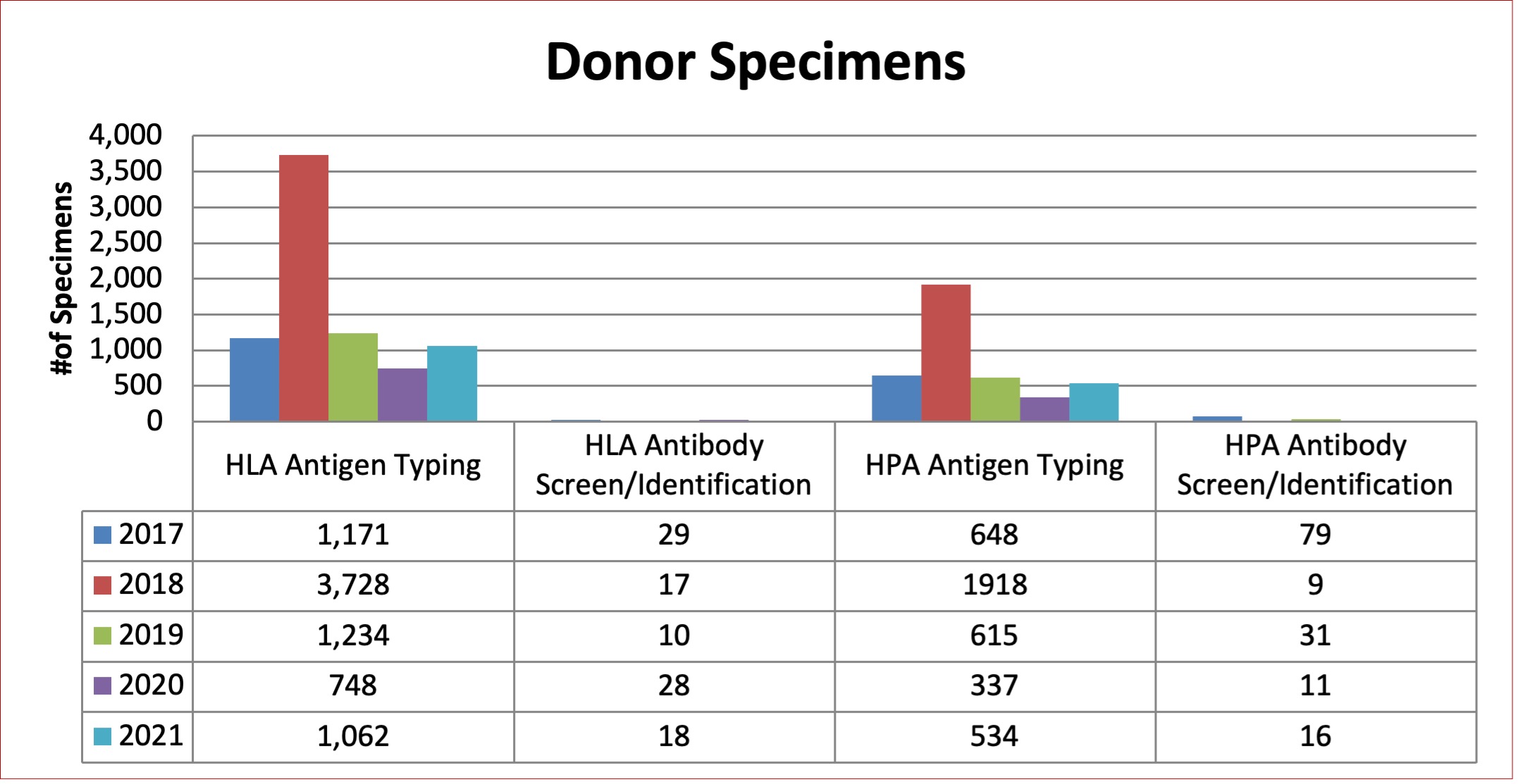
Figure 8: Total Platelet Immunology Patient Specimens Tested between 2017 and 2021
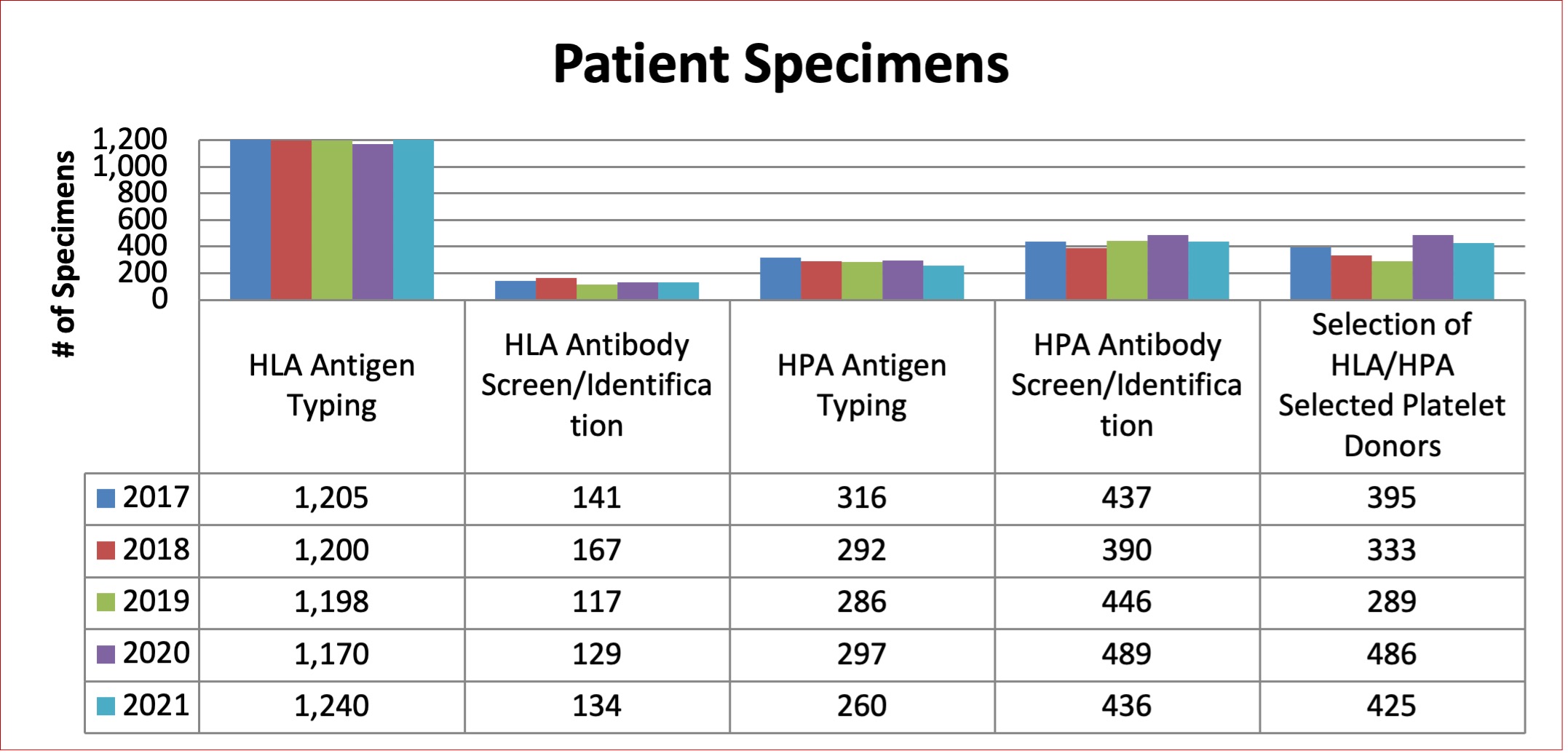
Table 9: Platelet Immunology Tests Performed
|
Specimen Type |
|
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|---|
|
Donor |
HLA Antigen Typing |
1,171 |
3,728 |
1,234 |
748 |
1,062 |
|
HLA Antibody Screen/Identification |
29 |
17 |
10 |
28 |
18 |
|
|
HPA Antigen Typing |
648 |
1918 |
615 |
337 |
534 |
|
|
HPA Antibody Screen/Identification |
79 |
9 |
31 |
11 |
16 |
|
|
Test Totals |
1,927 |
5,672 |
1,890 |
1,124 |
1,630 |
|
|
Patient |
HLA Antigen Typing |
1,205 |
1,200 |
1,198 |
1,170 |
1,240 |
|
HLA Antibody Screen/Identification |
141 |
167 |
117 |
129 |
134 |
|
|
316 |
292 |
286 |
297 |
260 |
||
|
HPA Antibody Screen/Identification |
437 |
390 |
446 |
489 |
436 |
|
|
Selection of HLA/HPA Selected Platelet Donors |
395 |
333 |
289 |
486 |
425 |
|
|
Test Totals |
2,494 |
2,382 |
2,336 |
2,571 |
2,495 |
|
Red Cell Genotyping
Canadian Blood Services is able to provide red cell antigen genotyping services through our National Immunohematology Reference Laboratory (NIRL) and Edmonton Diagnostic Services Laboratory. This service is used to aid in resolving complex immunohematology cases. Molecular testing combined with hemagglutination testing can provide better resolution to serological problems and guide patient transfusion requirements in some circumstances especially for sickle cell patients and patients with chronic transfusion requirements and multiple or complex antibodies.
Based on the following testing algorithm patients with serologically variable Rh D typing results may require genetic testing for the RHD gene.
Figure 9: Rh D Testing Algorithm
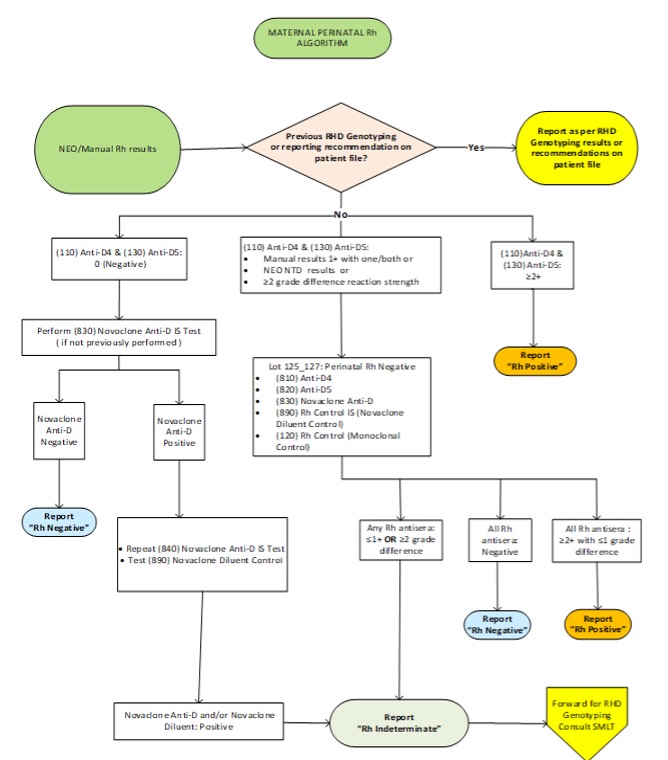
Text Version – Figure 9
Figure 9 describes maternal perinatal RhD testing algorithm. If NEO/Manual Rh results and previous RHD Genotyping results or previous recommendation are available on patient file, report as per RhD Genotyping results or recommendations on patient file.
If NEO/Manual Rh results are available but previous RHD Genotyping results or reporting recommendation on patient file are not available and Anti-D4 & Anti-D5 results are negative, check for the previous Novaclone Anti-D result. If previous Novaclone Anti-D Immediate Spin (IS) result is not available, perform Novaclone Anti-D IS test. If Novaclone Anti-D is Negative, report RhD as Rh Negative. If Novaclone Anti-D is positive, repeat Novaclone Anti-D IS Test and test Novaclone Diluent Control. If Novaclone Anti-D and/or Novaclone Diluent is Positive, report as Rh Indeterminate and forward the sample for RHD Genotyping and consult SMLT.
If NEO/Manual Rh results are available but previous RHD Genotyping results or reporting recommendation on patient file are not available and Anti-D4 & Anti-D5 manual results are 1+ with one or both antisera or NTD results with NEO or greater than or equal to two grade difference, perform tube tests for Anti-D4, Anti-D5, Monoclonal Control, Novaclone Anti-D and Novaclone Diluent Control. If any Rh Antisera results are less than or equal to one grade difference or greater than or equal to two grade difference, report as Rh Indeterminate and forward the sample for RHD Genotyping and consult SMLT. If all antisera results are negative, report RhD as Rh Negative. If all the Rh antisera results are greater than or equal to two with less than or equal to one grade difference, report RhD as Rh Positive.
If NEO/Manual Rh results are available but previous RHD Genotyping results or reporting recommendation on patient file are not available and Anti-D4 & Anti-D5 results are greater than or equal to two, report RhD as Rh Positive.
For 2021, the following results were obtained in patients using one of the two red cell antigen genotyping platforms available at CBS:
Table 10: Patient # - RHD Type/Result 2021
|
Patient |
RHD Genotype |
Predicted Phenotype |
RHD Sequencing |
Rh(D) Group |
|---|---|---|---|---|
|
1 |
Weak D type 2 |
Weak D |
RHD*01W.2 |
Positive |
|
2 |
Weak D type 1 |
Weak D |
RHD*01W.1 |
Positive |
|
3 |
Normal RHD |
D+ |
RHD*01/DIIIa-CE(4-7)-D |
Positive |
|
4 |
DAR |
|
RHD*09.01 |
Negative |
|
5 |
Weak D type 3 |
Weak D |
RHD*01W.3 |
Positive |
|
6 |
Normal RHD |
D+ |
RHD*01 |
Positive |
|
7 |
Normal RHD |
D+ |
RHD*01 |
Positive |
|
8 |
Weak D type 1 |
Weak D |
RHD*01W.1 |
Positive |
|
9 |
DAU4 DV type 5 |
|
RHD*10.04 RHD*05.05/DIIIa-CE(4-7)-D |
Negative |
|
10 |
Normal RHD |
D+ |
RHD*01 RHD-10.03 |
Positive |
|
11 |
Weak D type 3 |
Weak D |
RHD*01W.3 |
Positive |
|
12 |
RHD Deletion with DVII.1 |
|
RHD*01N.01 with RHD*07*01 |
Negative |
|
13 |
Weak D type 3 |
Weak D |
RHD*01W.3 |
Positive |
|
14 |
Weak D type 2 |
Weak D |
RHD*01W.2 |
Positive |
|
15 |
Normal RHD |
D+ |
RHD*01 |
Positive |
|
Normal RHD |
D+ |
RHD*01 |
Positive |
|
|
17 |
Weak D type 3 |
Weak D |
RHD*01W.3 |
Positive |
|
18 |
DAR |
|
RHD*09.01 |
Negative |
|
Weak D type 1 |
Weak D |
RHD*01W.1 |
Positive |
Quality Indicators
The laboratories monitor many quality indicators and the two which are most relevant to this document are turnaround times and rejected specimens which are presented below.
Turnaround Times
To ensure timely reporting of patient test results, Canadian Blood Services monitors turnaround time (TAT) from when the specimen is received at Canadian Blood Services in Winnipeg to the time when the results are available. Since monitoring of this quality indicator began in 2008, the percentage of specimens has consistently exceeded the predefined TAT threshold. Samples whose testing exceeds the expected TAT are usually those where clinically significant antibodies are detected or where difficulty in finding compatible blood is encountered.
Table 11: Turnaround Time – Routine Criteria by Specimen Type
Table 12: Turnaround Time – Routine Perinatal Specimens between 2017 and 2021
|
2017 |
2018 |
2019 |
2020 |
2021 |
|
|---|---|---|---|---|---|
|
% of Specimens Tested within 72 hours |
94% |
95% |
92% |
87% |
88% |
|
% of Specimens Tested > 72 hours |
6% |
5% |
8% |
13% |
12% |
Table 13: Turnaround Time – Routine Crossmatch Specimens between 2017 and 2021
|
2017 |
2018 |
2019 |
2020 |
2021 |
|
|---|---|---|---|---|---|
|
% of Specimens Tested within 24 hours |
100% |
99% |
99% |
99% |
99% |
|
% of Specimens Tested > 24 hours |
0% |
1% |
1% |
1% |
1% |
Table 14: Turnaround Time – Reference Specimens between 2017 and 2021
|
2017 |
2018 |
2019 |
2020 |
2021 |
|
|---|---|---|---|---|---|
|
% of Specimens Tested within 72 hours |
99% |
99% |
97% |
100% |
99% |
|
% of Specimens Tested > 72 hours |
1% |
1% |
3% |
0% |
1% |
Table 15: Turnaround Time - Platelet Immunology Specimens between 2017 and 2021
|
Turnaround Time (TAT) |
2017 |
2018 |
2019 |
2020 |
|
|---|---|---|---|---|---|
|
% of Specimens Tested within 14 days |
85.3%* |
97% |
99% |
98% |
98% |
|
% of Specimens Tested within 28 days |
95.30% |
94% |
98% |
98% |
99% |
|
% of Specimens Tested within 60 days |
91% |
99% |
92% |
100% |
100% |
* Preliminary results reported within 1-2 days of sample receipt.
Rejected Specimens
Each time a specimen is rejected, a reason for rejection is entered into our laboratory information system (LIS). This data is then retrieved and analyzed on a quarterly basis.
As described in Table 16 and Figure 10, the reasons for rejecting specimens in the Perinatal Laboratory are primarily problems with specimen labelling, requisitions and discrepancies between the requisition and the specimen. Average rejection rates have decreased from a high of 4.4% in 2012 to 3.4% in 2021 which correlates with increased efforts to contact customers and educate them on acceptable labelling criteria.
Table 17 and Figure 11 describe the reasons for rejecting specimens in the Crossmatch Laboratory; the majority of which involve problems with specimens. Problems with specimen labelling and discrepancies between the requisition and the specimen tube label constitute the main reasons for specimen rejection. Missing or incorrect information on the label and discrepancies in the name, personal health number (PHN) or date of collection continue to be the most common specimen labelling errors seen. Specimens are also rejected if the sample is a duplicate. The rejection rate for crossmatch specimens continued to remain low throughout 2021. The average rejection rates have decreased from a high of 2.9% in 2012 to 1.8% in 2021.
The rejection rates for perinatal specimens are higher than for crossmatch (pre-transfusion) specimens. The collection process for crossmatch specimens is controlled with stringent best practices and standards that must be followed. Crossmatch specimens are usually collected in hospitals and are sent to Canadian Blood Services via the hospital blood banks where the samples are pre-screened to determine if there are discrepancies between the sample and requisition. Perinatal specimens are most often collected in clinics and community collection sites where the identification and labelling process may be more variable. Although there may be differences in the collection process all specimens are scrutinized using the same stringent acceptance criteria prior to testing at Canadian Blood Services.
Some specimens for crossmatch have already been rejected by the referring hospital laboratory and total numbers of these rejected specimens are not included in our data.
Table 18 and Figure 12 describe the reasons for rejecting specimens in the Platelet Immunology Laboratory; the majority of which involve specimens. Samples may be rejected because of discrepancies between the specimen and the requisition, they are duplicate specimens that would not be tested; wrong tube type are all common reasons. The numbers represent an average rejection rate between both donor and patient rejections. Efforts to educate hospital customers continued throughout 2020 and 2021.
Figure 10: Rejection Reasons – Perinatal Specimens 2021
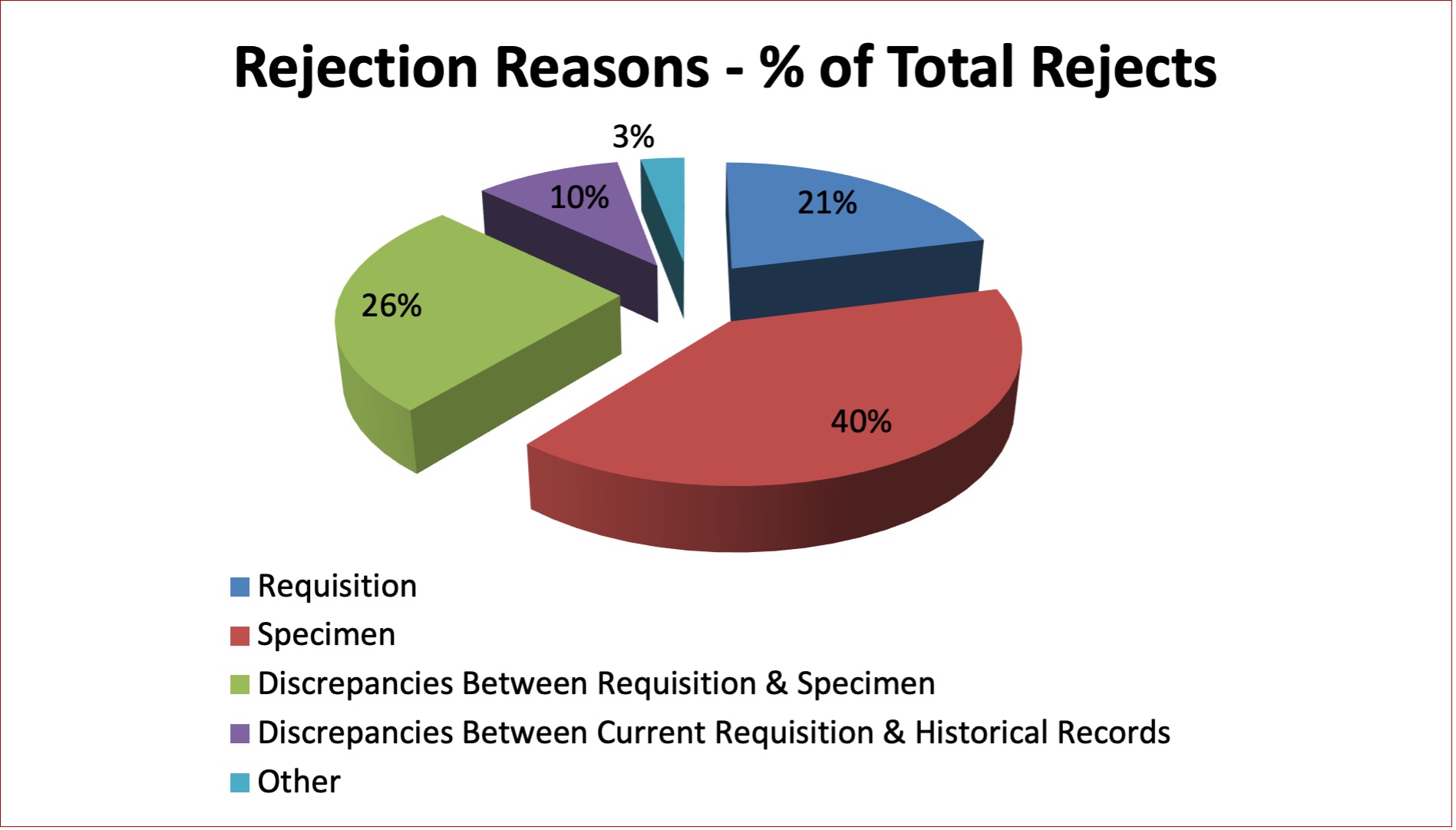
Table 16: Quarterly Rejection Rates – Perinatal Specimens 2021
|
Rejection Category |
% of Total Rejects |
Q1 |
Q2 |
Q3 |
Q4 |
|---|---|---|---|---|---|
|
Requisition |
21% |
77 |
46 |
39 |
49 |
|
Specimen |
40% |
117 |
95 |
92 |
94 |
|
Discrepancies Between Requisition & Specimen |
26% |
81 |
60 |
56 |
65 |
|
Discrepancies Between Current Requisition & Historical Records |
10% |
28 |
16 |
36 |
20 |
|
3% |
8 |
9 |
7 |
6 |
|
|
Total # specimens rejected |
|
311 |
226 |
230 |
234 |
|
Total # specimens received |
|
8,401 |
7,090 |
6,506 |
7,178 |
|
Rejections as a % of total |
|
3.7% |
3.2% |
3.5% |
3.3% |
Figure 11: Rejection Reasons – Crossmatch Specimens 2021
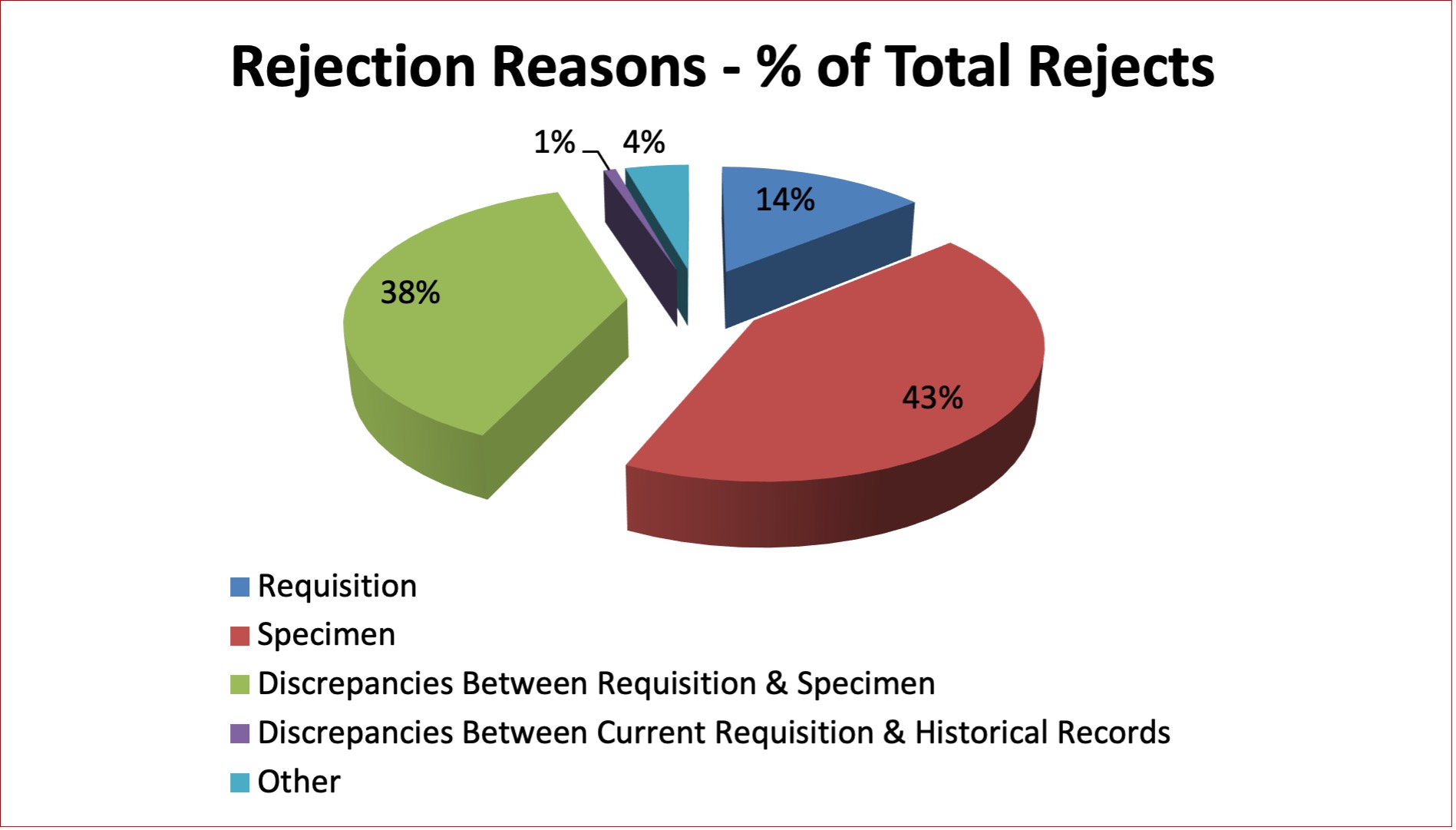
Table 17: Quarterly Rejection Rates – Crossmatch Specimens 2021
|
Rejection Category |
% of Total Rejects |
Q1 |
Q2 |
Q3 |
Q4 |
|---|---|---|---|---|---|
|
Requisition |
14% |
28 |
30 |
44 |
31 |
|
Specimen |
43% |
87 |
109 |
109 |
96 |
|
Discrepancies Between Requisition & Specimen |
38% |
71 |
85 |
110 |
92 |
|
Discrepancies Between Current Requisition & Historical Records |
1% |
0 |
1 |
3 |
4 |
|
4% |
10 |
12 |
9 |
10 |
|
|
Total # specimens rejected |
|
196 |
237 |
275 |
233 |
|
Total # specimens received |
|
8,858 |
8,665 |
8,897 |
25,576 |
|
Rejections as a % of total |
|
2.2% |
2.7% |
3.1% |
0.9% |
Figure 12: Platelet Immunology Rejection Reasons in the Year 2021

Table 18: Quarterly Rejection Rates – Platelet Immunology Specimens (Patient and Donor) 2021
|
Rejection Category |
% of Total Rejects |
Q1 |
Q2 |
Q3 |
Q4 |
|---|---|---|---|---|---|
|
Requisition |
8% |
1 |
5 |
3 |
5 |
|
Specimen |
75% |
24 |
30 |
29 |
46 |
|
Discrepancies Between Requisition & Specimen |
11% |
3 |
3 |
7 |
5 |
|
Discrepancies Between Current Requisition & Historical Records |
3% |
0 |
2 |
3 |
0 |
|
Unable to Enter Results in PROGESA |
1% |
0 |
1 |
1 |
0 |
|
2% |
0 |
3 |
1 |
0 |
|
|
Total # specimens rejected |
|
45 |
44 |
44 |
59 |
|
Total # specimens received |
|
571 |
614 |
564 |
645 |
|
Rejections as a % of total |
|
7.9% |
7.2% |
7.8% |
9.1% |
Diagnostic Services Update 2021
Updates pertain to all Diagnostic Services sites within Canadian Blood Services: Vancouver, Edmonton, Winnipeg, and Brampton
|
ALL |
NEO Iris Analyzer implemented April to June 2021. Eight NEO Instruments in Diagnostic Services were replaced with the next generation NEO IRIS instrument. NEO Iris performs ABO/RH and antibody testing.
|
|
Edmonton |
Edmonton DS obtained the CPSA 4-year accreditation on 20021-02-25. |
|
Edmonton |
Transfer of HEA and RHCE genotype testing to Brampton, 2021-10-01 |
|
Vancouver |
Awarded CAP Accreditation Dec 2021. |
|
Vancouver |
CPSBC – DAP ISO 15189 Audit. ISO 15189 accreditation pending final acceptance. |
|
Winnipeg |
Preparation for implementation of the Canadian Blood Services satellite Lab at St Boniface Hospital in March 2022. The Lab will act as a contingency site for services delivered by Winnipeg Diagnostic Services. |
|
Implementation of equipment in NPIRL – Multisizer 3 (cell counter) and thermocyclers |
|
|
Winnipeg |
Management of supplies, inventory and testing to ensure provision of services are not impacted during supply chain issues experienced in a pandemic. |
|
Winnipeg |
eTraceLine environments (perinatal and Crossmatch) were merged to allow better efficiency and ease of use for the labs noe that staff are cross trained. |
|
Winnipeg |
Project to implement HistoTrac and replace the access database currently used as the LIS began in Winnipeg in 2021. Projected implementation is December 2022. |
Presentations / Abstracts / Publications Listing
|
Lhevinne Ciurcovich, Lynnette Beaudin, Arianne Fuellos, Balkar Gill, Ilona Resz, Debra Lane, Judith Hannon, Gwen Clarke, Melanie Bodnar. Comparison of Manual SIAT vs Automated Solid Phase Methodology for Perinatal Antibody Titration. Poster, CSTM 2021 |
|
Lhevinne Ciurcovich1, Sarah Manfredi2, Sarah Buchko2, Darlene Mueller2, Michelle Wong2, Mohammad Bahmanyar2, Matthew Yan1, Gwen Clarke1. Anti-Ina Implicated in Hemolytic Disease of the Fetus and Newborn in an Indigenous Woman. Poster, CSTM 2021
|
|
Lhevinne Ciurcovich, Gwen Clarke, Matthew Yan. A Case of ABO Chimerism in a Perinatal Patient. Poster, CSTM 2021 |
|
Lhevinne Ciurcovich. Cell-Free Fetal DNA Testing: Advantages, Challenges and Limitations. Presentation: Virtual Conference, 22nd Annual Education Day on Blood Transfusion Issues, 2021-09-24. |
|
Lhevinne Ciurcovich. Immunohematology Case Studies. Presentation: Immucor ImmuTECH Education Day (Virtual) 2021-05-05. |
|
Lynnette Beaudin, Dr. Lani Lieberman MD, FRCP, Fetal and neonatal alloimmune thrombocytopenia (FNAIT): Diagnosis, Investigation and Treatment. Presentation: U of T Monthly Transfusion Rounds (Virtual) 2021-02-25 |
|
Bodnar M, Hannaford K, Montemayor-Garcia C, Hannon J. Blindspots in Immucor BioArray RHD Molecular BeadChip Test: A Review of Cases at Canadian Blood Services Referred Out for RHD Gene Sequencing. Poster/Abstract, CSTM 2021 |
|
Floch A, Vege S, Berardi P, Hannon J, Ochoa-Garay G, Lomas-Francis C, et al. A change in RHD is associated with aberrant transcription and very weak D phenotype. Transfusion 2021 |
|
Flegel WA, Bodnar M, Clarke G, Hannon J, Lieberman L., ‘What constitutes the most cautious approach for a pregnant person with weak D type 4.0?’ Letter to the Editor, CMAJ June 2021 |